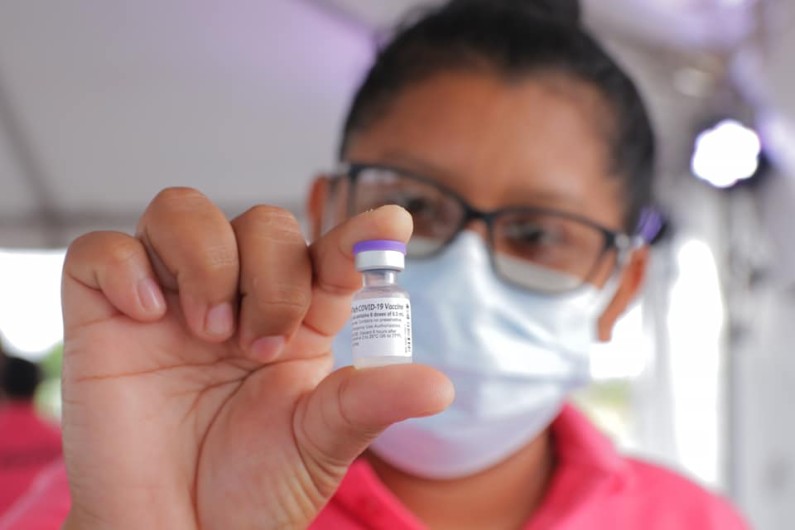
When the United States implements its COVID-19 vaccine requirement for all international arriving passengers from the 1st November, it will only recognise vaccines approved by the World Health Organisation or the US Food and Drug Administration.
The Russian-made Sputnik V vaccine which has been administered to over 175,000 persons in Guyana is still awaiting WHO approval and therefore persons administered with the vaccine will not be allowed to travel to the US.
The Reuters News agency quoted a US Centers for Disease Control Official as stating that “six vaccines that are FDA authorized/approved or listed for emergency use by WHO will meet the criteria for travel to the U.S.”
The official said the airlines have already been informed of the decision and the US-CDC continues to put systems in place to prepare for the new vaccination requirement for travel to the US.
The manufacturers of the Sputnik V vaccine are confident that the vaccine will receive WHO approval soon. While it was one of the first vaccines developed, it is still to receive approval by the World Health Organisation.
The United States recently also introduced COVID-19 vaccine requirement as part of the medical process for persons seeking permanent visa approvals for the US. Only US-FDA and WHO-approved vaccines are being recognised for that visa process.
Earlier this year, a number of international students who traveled to the US to attend university were forced to get revaccinated with a US-approved vaccine since many of the universities only recognise the US vaccines.
But the Pan American Health Organisation recently advised against revaccination with a shot that is different to the one used initially.
At a press conference, the PAHO chief Jarbas Barbosa said “we are not recommending getting different vaccines for any reason. There is no research that guarantees that this procedure is safe for those who had a different jab”.








You must be logged in to post a comment Login